Synthesis of a cousin of ultra-strong graphene
2 min read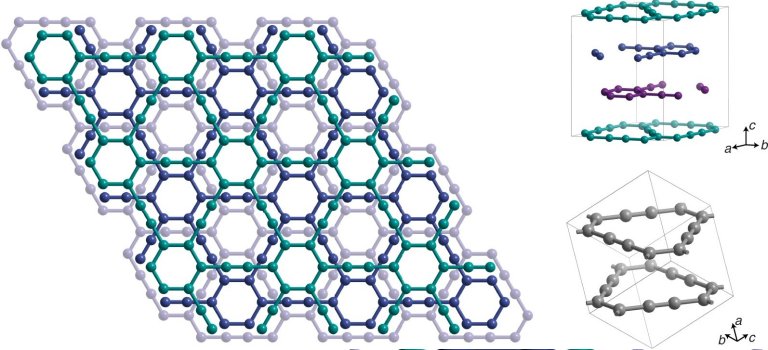
Nano technology
Technology Innovation Website Editor – 05/24/2022

Crystal structure of the graphene layer.
[Imagem: Yiming Hu]
graphene
After more than 10 years of trying, researchers have finally been able to synthesize graphene, a nanoscale carbon that can be even stronger than graphene.
“The whole audience, the entire field, is really excited because this long-standing problem, or this article, [longamen te] Professor Yiming Hu of the University of Colorado Boulder (USA) said:
While graphene is made of carbon atoms arranged in a honeycomb shape, forming a plate just one atom thick, graphene has a slightly more complex design, with hexagons connected as if they were the vertices of triangles.
“Unlike graphenes, which consist only of sp2-hybridized carbon, graphenes contain sp-hybridized carbon periodically incorporated into the sp2-hybridized carbon structure,” the team details.
There are different ways in which carbon trops can be built, depending on how sp2, sp3, sp hybridized carbon (or the different ways in which carbon atoms can bond to other elements) and their corresponding bonds are used.
The most famous types of carbon substitutes are graphite and diamond, which are created from sp2 carbon and sp3 carbon, respectively. However, especially after the discovery of graphene, there is now an infinity of so-called “nanocarbons”, including fullerenes, nanotubes, nanodiamonds, etc.

The next step will be to characterize the graphene samples, to confirm its control properties, which make it superior to graphene.
[Imagem: Yiming Hu et al. – 10.1038/s44160-022-00068-7]
Graphene’s superpowers
Graphene has been researched for a long time because theories suggest it has the same very high electrical conductivity as graphene, but with an essential addition for practical applications: the ability to control electric current.
“Grapene was expected to exhibit unique and interesting mechanical and optical properties of electron conduction. Specifically, the electron conduction in graphene should be exceptionally fast, as in graphene. However, in some graphene the electron conduction can be controlled. in a specific direction, such as in contrast to the multidirectional conduction in graphene, because triple bonds can create a distortion in Dirac conesTeam explanation.
He and his colleagues showed that they were able to synthesize graphene—specifically, γ-graphene, which is the most stable form of graphene—using a process called alkene reaction, an organic reaction that involves redistribution, or cutting, and reshaping of an alkene. Chemical bonds (a type of hydrocarbon with at least one carbon-carbon triple bond).
“There is a very big difference (between graphene and graphene), but in a good way. This could be a great material for the next generation. That’s why people are so excited,” said Professor Wei Zhang, coordinator of the team.
Article: Synthesis of γ-Graphyne using dynamic covalent chemistry
Authors: Yiming Hu, Chenyu Wu, Qingyan Pan, Yinghua Jin, Rui Lyu, Vikina Martinez, Shaofeng Huang, Jingyi Wu, Lacey J. Wayment, Noel A. Clark, Markus B. Raschke, Yingjie Zhao, Wei Zhang
Journal: Synthesis of Nature
DOI: 10.1038 / s44160-022-00068-7

Other news about:
More topics

“Entrepreneur. Music enthusiast. Lifelong communicator. General coffee aficionado. Internet scholar.”

:strip_icc()/s04.video.glbimg.com/x720/11792055.jpg)

:strip_icc()/s03.video.glbimg.com/x720/11786998.jpg)