A lithium-air battery stores four times more energy than lithium-ion currents
2 min read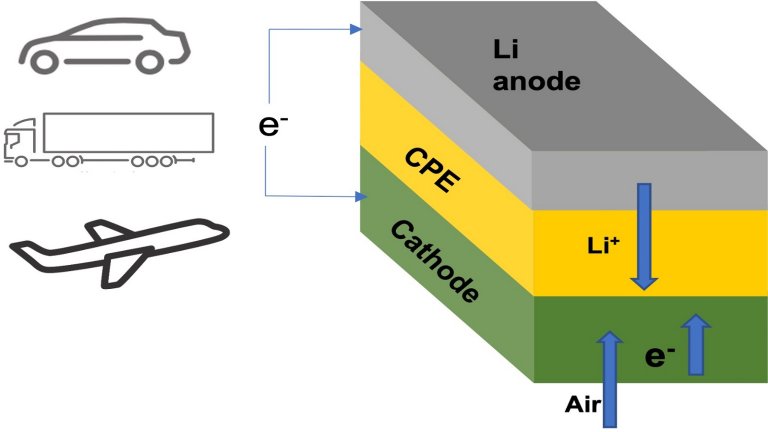
energy
Technology Innovation Website Editor – 07/03/2023

The schematic shows a lithium-air battery consisting of a lithium metal anode, an air-based cathode, and a solid ceramic polymer electrolyte (CPE).
[Imagem: ANL]
Lithium oxygen battery
Long-awaited lithium-oxygen batteries (Li-Otwo), or lithium-air, is so efficient that it promises to make electric trucks viable on long haul flights and even electric planes.
The main component of the new lithium-air battery is a solid electrolyte, not the usual liquid. Batteries with solid electrolytes are not subject to the safety issue of liquid electrolytes, which can overheat and catch fire. But its big gain lies in efficiency.
“The lithium-air battery has the highest design energy density of any battery technology considered for next-generation batteries other than lithium-ion,” said Larry Curtis of Argentine National Laboratory in the US.
Curtis and his colleagues have just developed a new structure and chemistry that can increase the energy density by up to four times that of current liquid lithium-ion batteries.
The secret to this lies in the lithium metal anode, the solid ceramic and polymer electrolyte, and the cathode which is the surrounding air itself.
First prototype lithium-oxygen battery that achieved a four-electron interaction at room temperature.
[Imagem: Alireza Kondori et al. – 10.1126/science.abq1347]
1000 charge and discharge cycles
In previous designs of lithium-oxygen batteries, lithium moves from the metal anode through a liquid electrolyte to combine with oxygen during discharge, producing lithium peroxide (Litwoatwo) or superoxide (LiOtwo) on the cathode. The lithium peroxide or superoxide then decomposes into lithium and oxygen components during charging. This chemical sequence stores and releases energy on demand.
The new solid electrolyte the team developed consists of a polymer-ceramic material, which is made of relatively inexpensive elements in the form of nanoparticles. This solid allows the chemical reactions that produce lithium oxide (Litwoo) When unloading.
“The chemical reaction of lithium superoxide, or peroxide, involves one or two stored electrons per oxygen molecule, while the lithium oxide reaction involves four electrons,” says team member Rashid Amin.
More stored electrons mean higher energy density. In fact, this is the first prototype lithium-oxygen battery that has achieved a stable interaction of four electrons at room temperature: the battery has exceeded 1,000 charge-discharge cycles, a mark considered benchmark for an experimental battery. .
“With further development, we expect our new lithium-air battery design to reach a record energy density of 1,200 watt-hours per kilogram,” Curtis said. “That’s nearly four times better than lithium-ion batteries.”
condition: Room temperature rechargeable Li2O-based lithium-air battery powered by a solid electrolyte
Authors: Alireza Konduri, Mohammadreza Ismailirad, Ahmad Mousin Harzandi, Rashid Amin, Mahmoud Tamadoni Saray, Li Yu, Tongchao Liu, Jianguo Wen, Nanan Shan, Hsin-Hao Wang, Anh Thi Ngoo, Paul C. Redfern, Christopher S Johnson Khalil Amin, Reza Shahbazian Yasar, Larry A. Curtis, Muhammad Asadi
Journal: Science
Volume: 379, Issue 6631 p. 499-505
DOI: 10.1126/science.abq1347
Other news about:
More topics

“Entrepreneur. Music enthusiast. Lifelong communicator. General coffee aficionado. Internet scholar.”


:strip_icc()/s04.video.glbimg.com/x720/11792055.jpg)

:strip_icc()/s03.video.glbimg.com/x720/11786998.jpg)